
Mathias Daniels
Shared posts
[ASAP] Synthesis of 1,3-Dioxepine-Fused (Tricyclic) Bispyrazoles Involved with Pyrazolone Derivatives and Dichloromethane
[ASAP] Catalytic Oxidative C–H Annulation of Arylthiol Derivatives with 1,3-Diynes toward 3,3′-Bibenzothiophenes

SupraFit - An Open source Qt based fitting application to determine stability constants from titration experiments
[ASAP] When Safety Data Sheets are a Safety Hazard

Carbonylation Chemistry Applied to the Synthesis of Benzimidazo[2,1‐b]quinazolin‐12‐ones
![Carbonylation Chemistry Applied to the Synthesis of Benzimidazo[2,1-b]quinazolin-12-ones](https://chemistry-europe.onlinelibrary.wiley.com/cms/asset/63d99c76-ccad-4487-a794-9b42e4586a39/ejoc202101136-toc-0001-m.png)
A new synthetic route towards benzimidazo[2,1-b]quinazolin-12-ones has been developed, which relies on the Pd-catalyzed intramolecular aminocarbonylation of N-(2-bromophenyl)-1H-benzimidazol-2-amines. Using near stoichiometric amounts of 13CO, isotopically labelled benzimidazo[2,1-b]quinazolin-12-ones were synthesized.
Abstract
A carbonylative route towards the synthesis of benzimidazo[2,1-b]quinazolin-12-ones was developed. The key step in this strategy consists of an intramolecular carbonylative lactam formation, starting from N-(2-bromophenyl)-1H-benzimidazol-2-amines. These precursor molecules were synthesized by two different methods to introduce a variety of substituents on the aromatic ring systems. Interestingly, only near-stoichiometric amounts of carbon monoxide were required in the ring-closing aminocarbonylation reaction, rendering the developed strategy also suitable for late-stage 13C-isotopic labelling.
[ASAP] TMS is Superior to Residual CHCl3 for Use as the Internal Reference for Routine 1H NMR Spectra Recorded in CDCl3

[ASAP] An Enzymatic Platform for Primary Amination of 1-Aryl-2-alkyl Alkynes

[ASAP] One-Shot Synthesis of Expanded Heterohelicene Exhibiting Narrowband Thermally Activated Delayed Fluorescence

[ASAP] Readily Reconfigurable Continuous-Stirred Tank Photochemical Reactor Platform

Enantioselective Synthesis of Dithia[5]helicenes and their Postsynthetic Functionalization to Access Dithia[9]helicenes
![Enantioselective Synthesis of Dithia[5]helicenes and their Postsynthetic Functionalization to Access Dithia[9]helicenes](https://onlinelibrary.wiley.com/cms/asset/77f62878-15db-4e75-a69e-c76681ecdff2/anie202114577-toc-0001-m.png)
The use of a chiral Au catalyst bearing an α-cationic phosphonite as an ancillary ligand and two successive alkyne hydroarylation events allows the assembly of dithia[5]helicenes with excellent enantioselectivity. Moreover, regioselective postsynthetic bromination of the dithia[5]helicene products generates starting materials for the efficient preparation of even more π-expanded systems, such as dithia[9]helicenes.
Abstract
A highly enantioselective synthesis of 5,13-disubstituted dibenzo[d,d′]benzo[1,2-b:4,3-b′]dithiophenes is reported. Key for the successful assembly of these helical architectures is the last two successive Au-catalyzed intramolecular alkyne hydroarylation events. Specifically, the second cyclization is the enantiodetermining step of the whole process and provides the desired helicenes with excellent ee values when a TADDOL-derived 1,2,3-(triazolium)phosphonite moiety (TADDOL: α,α,α′,α′-tetraaryl-1,3-dioxolane-4,5-dimethanol) is employed as an ancillary ligand. The absolute stereochemistry of the newly prepared structures has been determined by X-ray crystallography to be P; the optical properties of these heterohelicenes are also reported. A three-step procedure was subsequently developed that allows the transformation of the initially obtained dithia[5]helicenes into dithia[9]helicenes without erosion of the enantiopurity.
[ASAP] Infinitene: A Helically Twisted Figure-Eight [12]Circulene Topoisomer

[ASAP] Violations. How Nature Circumvents the Woodward–Hoffmann Rules and Promotes the Forbidden Conrotatory 4n + 2 Electron Electrocyclization of Prinzbach’s Vinylogous Sesquifulvalene

Does Nucleophilic Substitution in Nitroarenes Proceed via Single Electron Transfer (SET)?

The interaction of nucleophiles with nitroarenes can result in direct, reversible addition to form σ adducts and products of SNAr, or in single electron transfer (SET) which usually initiates other processes.
Abstract
Analysis of effects of various parameters on reactions of C, N and O nucleophiles with nitroarenes leads to the conclusion that there are two different major pathways: direct addition leading to substitution of hydrogen or halogens and single electron transfer (SET), leading to other processes. In some, rather rare cases, paramagnetic species generated by SET can combine, resulting in substitution.
A Journey through Hemetsberger–Knittel, Leimgruber–Batcho and Bartoli Reactions: Access to Several Hydroxy 5‐ and 6‐Azaindoles

Abstract
The preparation of various 5- and 6-azaindoles, heterocyclic structures that are frequently part of molecules in clinical development, and their monohydroxy analogues were described. Different strategies, relying on the de novo pyrrole ring formation, were investigated and, thanks to Hemetsberger–Knittel, Bartoli and Leimgruber–Batcho approaches, 4- and 7-monohydroxy 5- and 6-azaindoles were obtained. The crucial introduction of the oxygen atom was carried out from halogen derivatives, using nucleophilic substitution reactions under basic conditions with or without a copper catalyst. Some preliminary oxidation reactions have shown that it was yet not possible to synthesize the azaquinone indole structure from monohydroxy azaindole, using molecular oxygen in the presence of salcomine as a catalyst.
Macromolecular helicity induction and static helicity memory of poly(biphenylylacetylene)s bearing aromatic pendant groups and their use as chiral stationary phases for high‐performance liquid chromatography

Novel optically inactive poly(biphenylylacetylene)s bearing achiral aromatic ester pendants with different ester sequences were synthesized to develop helicity-memorized polymer-based chiral stationary phases for HPLC. An appropriately-designed one-handed helical poly(biphenylylacetylene) with the static helicity memory that was successfully produced based on the “macromolecular helicity induction and subsequent static helicity memory” strategy showed a remarkably high enantioseparation ability toward various kinds of chiral aromatics with point, axial, and planar chirality as well as helicity under the reversed-phase conditions.
Abstract
Two novel poly(biphenylylacetylene)s (PBPAs) bearing achiral alkylphenyl groups at the 4′-position of the biphenyl pendant through ester linkers with different sequences were synthesized by the rhodium-catalyzed polymerization of the corresponding monomers. The influence of the alkylphenyl pendants and the ester sequences on the macromolecular helicity induction and subsequent static helicity memory was investigated. In addition, the chiral recognition ability as chiral stationary phases for high-performance liquid chromatography of the helicity-memorized PBPAs was also examined. Both polymers formed almost perfect right- and left-handed helical conformations through noncovalent chiral interactions with enantiomeric alcohols, and their induced macromolecular helicities were completely retained (“memorized”) after removal of the helix inducer. A PBPA bearing a 4-n-butylphenoxycarbonyl pendant group with a static helicity memory showed a remarkably high chiral recognition ability toward a wide variety of chiral aromatics, including simple point chiral compounds, axially chiral biaryls, a chiral spiro compound, helicenes, and planar chiral cyclophanes, particularly under the reversed-phase conditions.
Identifying palladium culprits in amine catalysis
Nature Catalysis, Published online: 02 December 2021; doi:10.1038/s41929-021-00710-1
Identifying palladium culprits in amine catalysisRevisiting the amine-catalysed cross-coupling
Nature Catalysis, Published online: 02 December 2021; doi:10.1038/s41929-021-00709-8
Revisiting the amine-catalysed cross-couplingDoes Nucleophilic Substitution in Nitroarenes Proceed via Single Electron Transfer (SET)?

The interaction of nucleophiles with nitroarenes can result in direct, reversible addition to form σ adducts and products of SNAr, or in single electron transfer (SET) which usually initiates other processes.
Abstract
Analysis of effects of various parameters on reactions of C, N and O nucleophiles with nitroarenes leads to the conclusion that there are two different major pathways: direct addition leading to substitution of hydrogen or halogens and single electron transfer (SET), leading to other processes. In some, rather rare cases, paramagnetic species generated by SET can combine, resulting in substitution.
An Oxidant- and Catalyst-Free Synthesis of Dibenzo[a,c]carbazoles via UV Light Irradiation of 2,3-Diphenyl-1H-indoles
Synthesis
DOI: 10.1055/a-1677-4881

An efficient methodology for the synthesis of dibenzo[a,c]carbazoles via annulation of 2,3-diphenyl-1H-indoles in EtOH under UV light irradiation (λ = 365 nm) along with hydrogen evolution is described. This method exhibits the advantages of mild reaction conditions, no requirement of any oxidants and catalysts, and release of hydrogen as the only byproduct. Notably, the mechanism investigation confirms that the trans-4b,8a-dihydro-9H-dibenzo[a,c]carbazole intermediate could convert into cis-4b,8a-dihydro-9H-dibenzo[a,c]carbazole, which relies on the nitrogen atom of the indole ring. This is followed by intramolecular dehydrogenation which yields the dibenzo[a,c]carbazoles.
[...]
Georg Thieme Verlag KG Rüdigerstraße 14, 70469 Stuttgart, Germany
Article in Thieme eJournals:
Table of contents | Abstract | Full text
[ASAP] Nickel-Catalyzed Enantioselective Arylative Activation of Aromatic C–O Bond

[ASAP] Double π-Extended Helicene Derivatives Containing Pentagonal Rings: Synthesis, Crystal Analyses, and Photophysics

[ASAP] Cascade Reaction of 2-Naphthols and Azirines: One-Pot Synthesis of C-3 Naphthol-Substituted Benzo[e]indoles

[ASAP] Process Development, Manufacture, and Understanding of the Atropisomerism and Polymorphism of Verinurad

[ASAP] Influence of Solvents and Additives on the Pyrophoricity of Palladium on Carbon Catalyst after Hydrogenation

[ASAP] Ring-Expansion Strategy for α-Aryl Azahelicene Construction: Building Blocks for Optoelectronic Materials

[ASAP] Synthesis and Chiral Resolution of Twisted Carbon Nanobelts

A visible-light-induced, metal-free bis-arylation of 2,5-dichlorobenzoquinone
Abstract
A metal-free protocol for the direct bis-arylation of 2,5-dichlorobenzoquinone with aryldiazonium salts is reported. The reactive salts are generated in situ and converted to radicals through irradiation with visible light. Reaction products precipitate from the solvent, eliminating the need for purification and thus providing a novel green method for the synthesis of versatile bis-electrophiles.
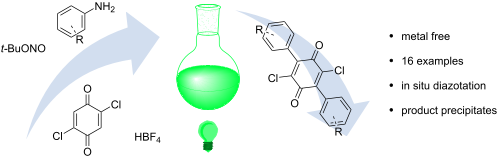
Beilstein J. Org. Chem. 2021, 17, 2315–2320. doi:10.3762/bjoc.17.149
Five keys to writing a reproducible lab protocol
Nature, Published online: 06 September 2021; doi:10.1038/d41586-021-02428-3
Effective sharing of experimental methods is crucial to ensuring that others can repeat results. An abundance of tools is available to help.[ASAP] S,C,C- and O,C,C-Bridged Triarylamines and Their Persistent Radical Cations

[ASAP] Synthesis of Saddle-Shape Octaaminotetraphenylene Octahydrochloride
